Service Detail
At Revive Medical Technologies, we take pride in our commitment to precision, reliability, and innovation. Our Quality Testing Service is your trusted partner in the pursuit of excellence, helping you achieve consistent quality, reduce operational risks, and ultimately, drive your business to new heights of success.






Our product development services are always focused on conformance to applicable international standards and relevant regulatory body. Our team has decades of experience in manufacturing of medical devices and has previously established a complete Quality Management System in accordance with the self-developed Quality Plan. We offer our clients support in development and implementation of a realistic Quality Plan to establish their Quality Management System for Medical Device Manufacturing.
One of the most essential components for establishing Quality Management System (QMS) for Medical Devices is Quality Plan. It highlights how the QMS would be implemented over for specific case (e.g., product manufacturing). A quality plan usually covers the processes and quality elements which are applicable over the specific case. It further includes internal and external requirements may not be necessary for the specific case but are included to ensure acceptability from external elements like end-user, local market norms, local regulatory bodies, etc. Quality Plan is also dependent upon how well the QMS documentation can support it.


Testing of products-under development in a simulated environment with aid of specialized equipment, tools and software, opens avenues for evaluating different performance and safety attributes which can be expressed qualitatively and quantitatively. We offer specialized bench testing solutions focused on testing our client’s under-development products.
It is the imitation of the operation of a real-world process or system over time. Simulations require the use of models; the model represents the key characteristics or behaviors of the selected system or process, whereas the simulation represents the evolution of the model over time. We provide advanced computation modelling (e.g., Finite Element Analysis) to simulate working conditions to predict behavior of an under-development product.


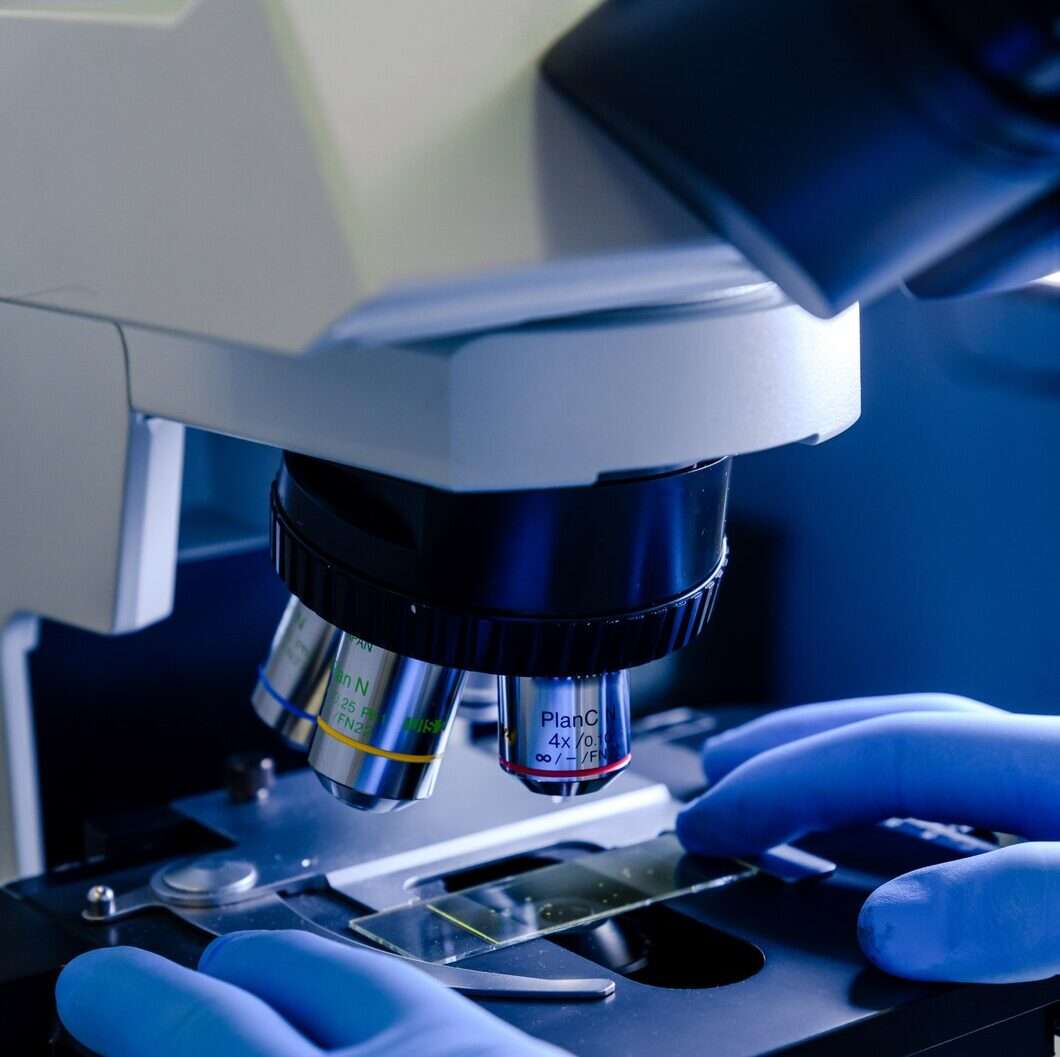
We offer services for Visual Inspection which is a globally employed quality inspection technique. Our team of highly skilled and experienced scientists and engineers’ using high quality visual inspection technology like high-magnification digital microscopes can visually detect the smallest defects or irregularities in a product which may have an impact on the product’s performance or safety.
Here at Revive Medical Technologies, our team of highly skilled engineers & developers offer services for dimensional analysis of our client’s products using high precision calibrated dimensional inspection equipment and tools.


This is defined as the study of properties, characteristics and impact of product-based defects. We provide services for Defect Analysis where our experienced team will conduct in-depth assessment of product defects, its origin and its impact over the product’s performance and safety.
We at Revive Medical Technologies offer services for conducting packing integrity testing for our client’s product where we identify packaging problems which may have an impact on the product and its sterility. We offer to conduct microbial challenge testing, dye penetration testing and seal integrity testing for evaluating packaging integrity of our client’s products.

Physicochemical testing of biomaterials and drug delivery systems involves a series of analytical and experimental techniques to assess and characterize the physical and chemical properties of these materials. These tests are crucial for ensuring the quality, safety, and effectiveness of biomaterials and drug delivery systems. Here are some common types of physicochemical testing that we offer:
Quotation
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis.